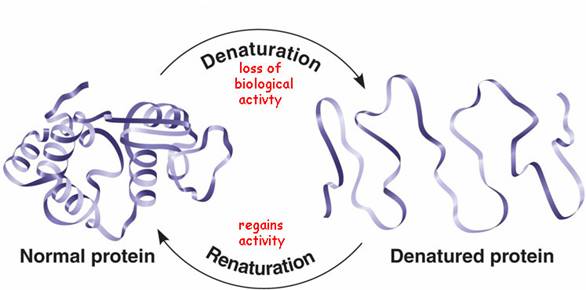
Protein denaturation
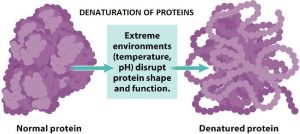
When a solution of a protein is boiled, the protein often becomes insoluble, that is, denatured, and remains insoluble even when the solution is cooled. Heat denaturation of egg white proteins, such as when boiling an egg, is an example of irreversible denaturation. The denatured protein has the same primary structure as the original or native protein. However, the weak forces between the charged groups and the weaker forces of the mutual attraction of the nonpolar groups break down at high temperatures; as a result, the tertiary structure of the protein is lost. In some cases, the original structure of the protein can be regenerated; the process is called renaturation.
Denaturation can be carried out in various ways. Proteins are denatured by treatment with alkaline or acidic agents, oxidizing or reducing agents, and certain organic solvents. Interesting the denaturing agents are those that affect the secondary and tertiary structure without affecting the primary structure. The most frequently used agents for this purpose are urea and guanidinium chloride. These molecules, due to their high affinity for peptide bonds, break the hydrogen bonds and salt bridges between the positive and negative side chains, thus abolishing the tertiary structure of the peptide chain.
When denaturing agents are removed from a protein solution, the native protein reforms in many cases. Denaturation can also be achieved by the reduction of cystine disulfide bonds, that is, conversion of the disulfide bond (―S―S―) into two sulfhydryl groups (―SH). This, of course, results in the formation of two cysteines. Reoxidation of cysteines by exposure to air sometimes regenerates native protein. In other cases, however, the wrong cysteines bind to each other, resulting in a different protein. Finally, denaturation can also be achieved by exposing proteins to organic solvents such as ethanol or acetone. Organic solvents are believed to interfere with the mutual attraction of nonpolar groups.
However, some of the smaller proteins are extremely stable, even in the face of heat; for example, ribonuclease solutions can be exposed for short periods to temperatures of 90°C (194°F) without undergoing significant denaturation. Denaturation does not involve identical changes in protein molecules. However, a common property of denatured proteins is the loss of biological activity, for example, the ability to act as enzymes or hormones. Although denaturation has long been considered an all-or-nothing reaction, it is now believed that many intermediate states exist between native and denatured protein. In some cases, however, the breaking of a key bond could be followed by the complete breaking of the native protein conformation.
Although many native proteins are resistant to the action of the enzyme trypsin, which breaks down proteins during digestion, they are hydrolyzed by the same enzyme after denaturation. Peptide bonds that can be cleaved by trypsin are inaccessible in native proteins but become accessible during denaturation. Similarly, denatured proteins give more intense colour reactions for tyrosine, histidine, and arginine than the same proteins in the native state. The increased accessibility of reactive groups on denatured proteins is attributed to an unfolding of the peptide chains.
If denaturation can be carried out easily and if renaturation is difficult, how is the native conformation of globular proteins maintained in living organisms, where they are produced step by step, incorporating one amino acid at a time? Experiments on the biosynthesis of proteins from amino acids containing radioactive carbon or heavy hydrogen reveal that the protein molecule gradually grows from the N-terminus to the C-terminus; a single amino acid residue is incorporated in each step. As soon as the growing peptide chain contains six or seven amino acid residues, the side chains interact with each other and thus cause deviations from the straight-chain or β configuration. Depending on the nature of the side chains, this can result in the formation of an α-helix or loops closed by hydrogen bonds or disulfide bridges. The final confirmation is probably frozen when the peptide chain reaches a length of 50 or more amino acid residues.
Confirmation of proteins at interfaces
Like many other substances with hydrophilic and hydrophobic groups, soluble proteins tend to migrate at the interface between air and water or oil and water; the term oil here means a hydrophobic liquid such as benzene or xylene. Within the interface, the proteins spread out, forming thin films. Measurements of the surface tension, or interfacial tension, of such films, indicate that the protein film reduces tension. Proteins, when forming an interfacial film, are present as a monomolecular layer, that is, a layer one molecule high. Although globular protein molecules were once thought to fully unfold at the interface, it has now been established that many proteins can be recovered from films in their native state.
The application of lateral pressure on a protein film causes it to thicken and eventually form a layer with a height corresponding to the diameter of the native protein molecule. The protein molecules at an interface, due to Brownian motions (molecular vibrations), take up much more space than those in the film after the application of pressure. The Brownian motion of the compressed molecules is limited to the two dimensions of the interface since the protein molecules cannot move up or down. The movement of protein molecules at the air-water interface has been used to determine the molecular weight of proteins. The technique consists of measuring the force exerted by the protein layer on a barrier.
When a protein solution is shaken vigorously in the air, it forms a foam, because the soluble proteins migrate to the air-water interface and persist there, preventing or slowing down the reconversion of the foam into a homogeneous solution. Some of the unstable and easily modifiable proteins denature when they spread out at the air-water interface. The formation of a permanent foam when the egg white is shaken vigorously is an example of irreversible denaturation when spread on a surface.